Isotopes have many applications in the environment. For example, the isotopes of carbon (14C) are widely used in recent dating. Oxygen (18O) isotopes can be used to reconstruct climates and temperatures when analyzed in ice or the shells of small marine animals.
Tritium, the hydrogen isotope seen in the previous lesson, can be used to track water masses in groundwater and thus estimate groundwater recharge times.
As for stable isotopes of metals and metalloids, they can generally be used to trace the different sources of contamination and the processes that can impact the mobility or toxicity of these elements. These traces are important in the context of the protection of populations or the environment, most of these metals being toxic and are emitted mainly by mining, industrial activities or urban environments.
In the context of remediation, the use of isotopes makes it possible to precisely target the problematic source and thus be more effective and less expensive than conventional remediation.
I. Isotopic tracing of contamination sources in a nutshell
All the elements are naturally present in the environment, at more or less high concentrations depending on the location, this is called the “geochemical background”.
This geochemical background has an isotopic signature value, and in the case of a single contamination, the principle of isotopic tracing is relatively simple.
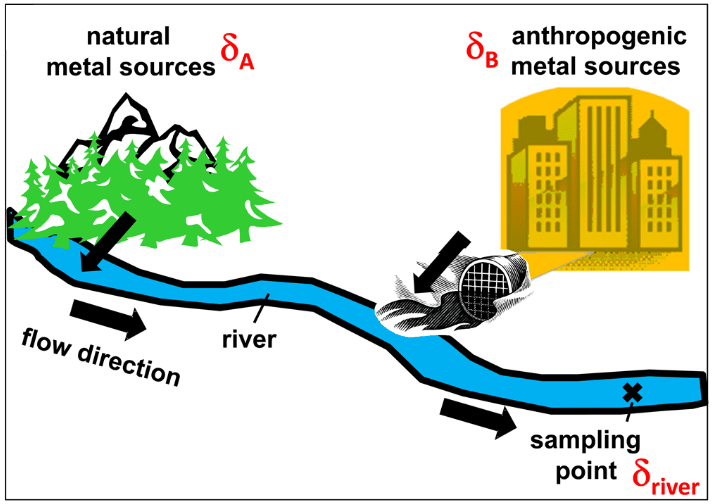
Diagram of a binary mixture between a geochemical background with δA signature and anthropogenic pollution with δB signature. From Wiederhold 2015.
In this case, where there is only anthropogenic pollution, the isotopic signature of the river will be the weighted average of each source. This average signature (δriver) is written in the form:

Equation of the isotopic mixture between two distinct sources whose proportions would be known.
Where δriver is the final isotopic signature of the river, fA the fraction (expressed in %) of the geochemical background relative to the total element concentration, and fB (in % too) the fraction of the anthropogenic concentration.
I. 1 Exemple
Let’s go back to our previous case, and let’s assume that our geochemical background has an isotopic signature of -1‰ while the contamination from the city has a concentration 4 times greater than the geochemical background with an isotopic signature of +1‰. What would be the final isotopic signature of the river?

Diagram of the isotopic mixture where the concentration of anthropogenic contamination is 4 times higher than the geochemical background. The values of -1 and +1 ‰ at the poles correspond respectively to the isotopic signature of the geochemical background and of the pollution.
In this case, the geochemical background fraction represents 20% of the total concentration of the element in the river. The fraction of pollution, meanwhile, represents 80% of the total.
So we have :
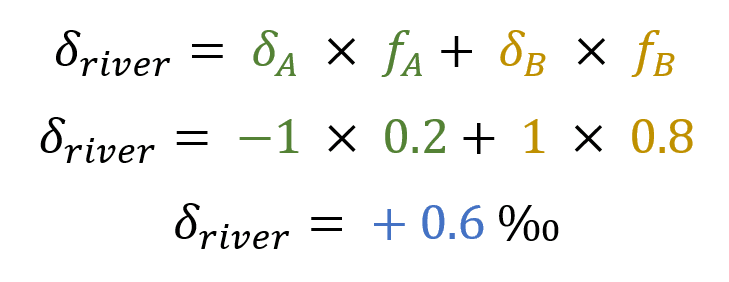
Detail of isotopic calculations of a binary mixture between a geochemical background and a source of pollution.
The final isotopic signature of the river would therefore be +0.6‰.
I. 2 Isotopic tracing in a complex environment
In a perfect theoretical case, isotopic tracing of sources is relatively straightforward. However, in complex and real environments, many processes can fractionate metal isotopes, i.e. change its isotopic signature. These processes can be for example redox changes, adsorption, precipitation…
These processes must be studied under experimental conditions and in isolation to know the extent of the resulting isotopic fractionation. The next lessons will focus on different ways to study these processes and apply them to a complex environment.