Isotopes are very effective tools in environmental studies, particularly to trace pollutions. Before we look at its various applications and uses, it is important to look at the basics: what is an isotope?
I. What is an atom ?
Atoms, which constitute matter, are mainly composed of empty space (more than 99%). However, they are composed of different elements:
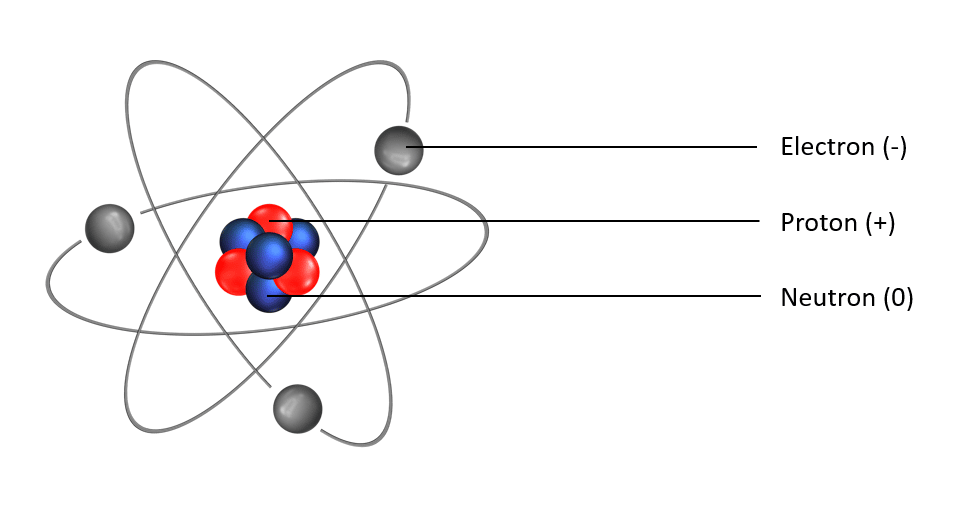
- Protons : particles which compose the atomic core and which possess a positive charge.
- Neutrons : also particles which compose the atomic core jointly with protons, but neutrons do not possess any charge. Protons and neutrons have virtually the same mass.
- Electrons : are forming an electronical cloud around the atomic core with smaller particles negatively charged. In an atom, the number of electrons ( – ) and protons ( + ) are similar, to form a neutral entity.
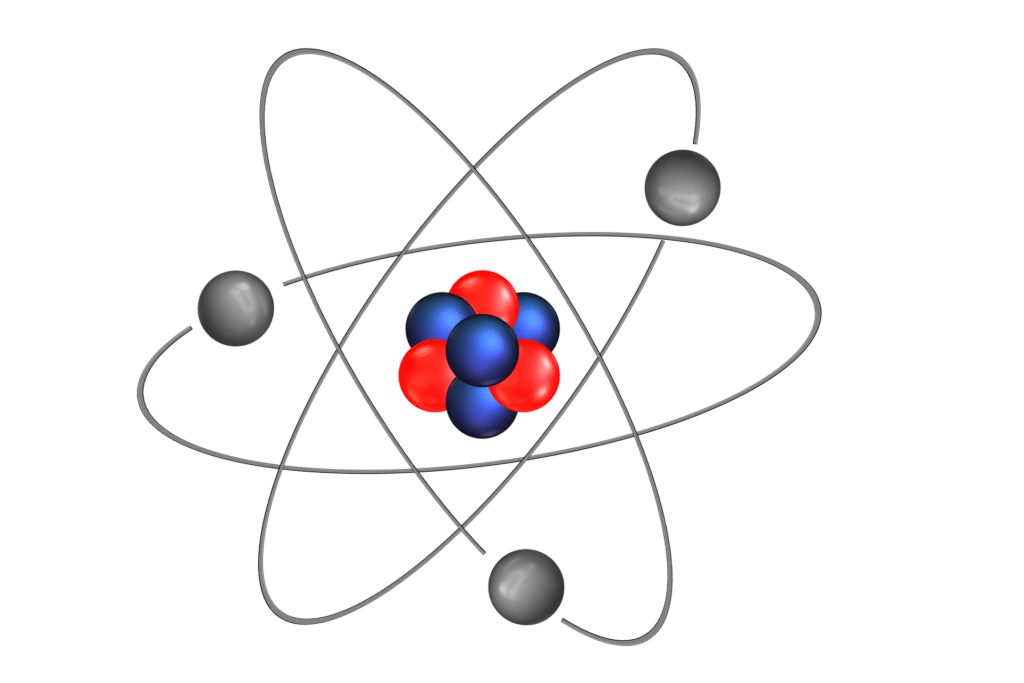
Scheme of an atom. In grey, are the electrons gravitating around the core, which is constituted of protons (in red) and neutrons (in blue).
How to read the periodic table of the element, exemple with the oxygen atom. The element symbol correspond to its english, french or latin name. The atomic number corresponds to the number of electrons and protons the element has. In the case of the oxygen, this number is equal to 8.
The atomic mass is the average mass of all the atoms of the same element, and corresponds for the oxygen atom of, in average, the weight of 8 protons + 8 neutrons (the weight of the electrons is negligible).
II. Isotopes... What are they ?
Out of almost 4000 known nuclides (natural and artificial), only about 287 are stable or have a very long lifespan (130Te (Tellurium) for example has a half-life of the order of 8.2×1020 years), and they correspond to 118 elements.
Are called “isotopes” the nuclides of the same element but which have different masses, due to a variation in the number of neutrons in the nucleus (the number of protons is identical). The nature of the chemical element is conditioned by its number of prontons and electrons (therefore the number of charges and electronic shells that an atom has), but is not influenced by the number of neutrons. The latter therefore vary slightly in number and spatially between the different atoms of the same element, and can influence its mass or its stability, but not its nature.
Isotope literally means “same place” and therefore refers to the different nuclides that are located in the same place on the periodic table of elements. The concept of isotope has been known since 1913, when Thomson used a cathode ray tube to determine the charge/mass (q/m) ratio of Neon, and discovered that the particles composing it had two masses, one of 20 which was known and the other of 22 which he first confused with a CO2 molecule with two charges. There are different types of isotopes (e.g. stable, radioactive, primordial, radiogenic, etc.). We will focus in using stable and non-radiogenic elements for source and processes tracing for the rest of these courses.
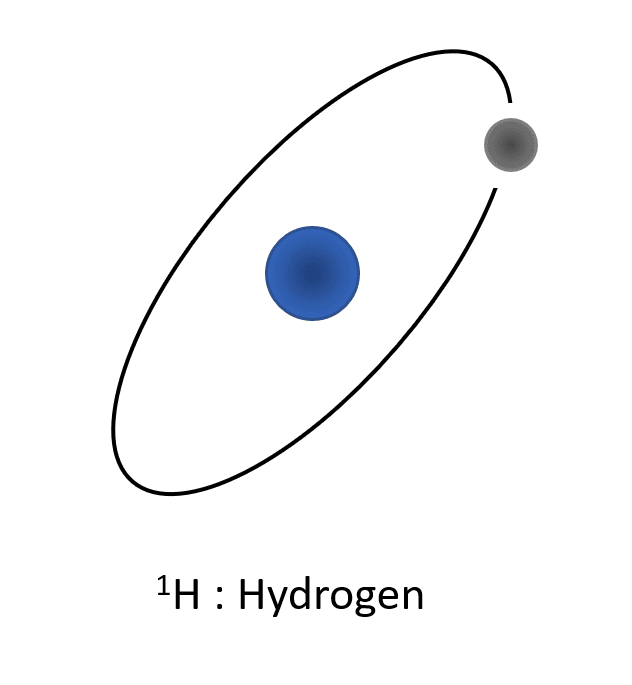
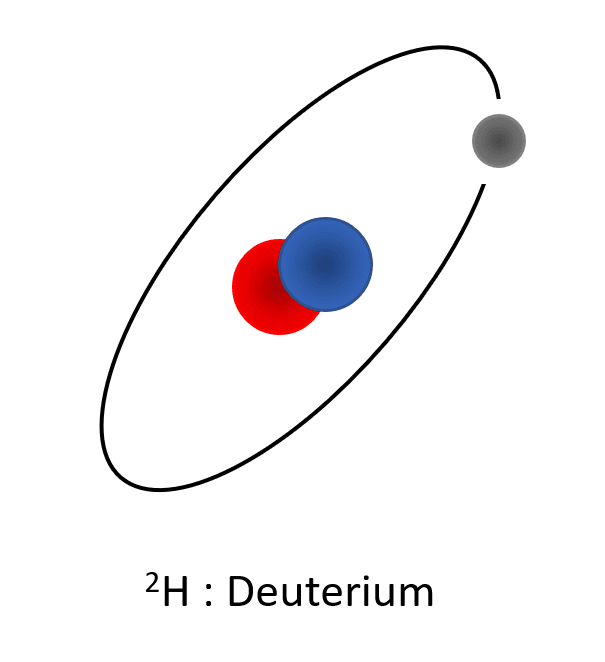
For exemple, in the case of the atom of hydrogen, the simplest, we have different isotopes :
- 1H composed of one proton and one electron. The atomic mass of this atom of hydrogen is 1 a.u. (atomic unit).
- 2H (deuterium) composed of one proton, one electron and one neutron. Its atomic mass is 2 a.u.
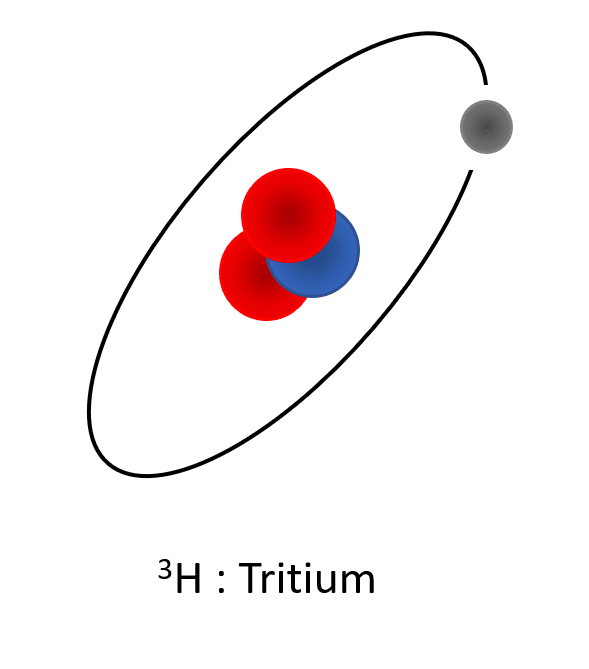
3H (tritium) composed of one proton, one electron and two neutrons. Its atomic mass is 3 a.u., and due to its charge repartition in its core, is instable and a marker from human nuclear activities.
Representation of the different isotopes of the hydrogen. Protons are represented in blue (positive charges in the core), the electrons are represented in grey (negative charges turning around the core) and neutrons are represented in red (no charges, localized in the core). All these isotopes have the same number of charges (1 proton and 1 electron), but differ in the number of neutrons which make the atomic weight different.
III. How to measure isotopes ?
There are different ways to measure isotopes, depending on their nature, size or concentration. Nowadays, most isotopic measurements are made on mass spectrometers, using magnetic and electric fields and are very present in medicine, chemistry or process control. These devices are able to measure most elements and allow several modes of introduction (generation of hydride, liquid introduction, laser ablation or desolvation).
The principle of the mass spectrometer is to separate the different atoms according to their mass using a magnet to send them into different Faraday cages which will, for each atom impact, transform the signal into an electrical signal, proportional to the number of atoms.
Example of how a mass spectrometer works. Here it is the MC-ICPMS Neptune plus (Thermofisher documentation).
The Multi-Collector ICP-MS has filled the void in accurate isotopic measurement between ICP-MS and Thermal Ionization Mass Spectrometer (TIMS) for the measurement of metallic elements. The term ICP means, in English “Inductively Coupled Plasma”, it is an argon plasma where the ionization of the elements takes place. The sample, in liquid or gaseous form, is sent to a nebulization chamber and is propelled by an argon gas through a plasma to be transformed into ions. The nebulizer serves to prevent as much as possible the injection of solvent on the plasma torch, thus maximizing its temperature and its stability, and at the same time limiting possible interference with other elements. These ions are then propelled through two cones (“sample” and “skimmer”) to finally recover only a beam of parallel ions.
Natural variations in isotopic ratios of stable isotopes of metals and metalloids are small. Thus, their isotopic composition is expressed with the notation δ (in ‰) which corresponds to the deviation of the isotopic ratio of a sample compared to that of a reference.
Exemple of the equation of the δ123Sb for the antimony (Sb) isotopic system which possesses two stable isotopes (121Sb for the light isotope and 123Sb for the heavy isotope).
As the values of the isotopic signatures are expressed in relation to isotopic standards, it is important in order to be able to inter-compare the values of samples between different teams to have a common or similar isotopic standard.
Now that we have seen the basics of isotopy, we will see in the next chapters what isotopic fractionation is, the different ways of studying the isotopes of each element, and finally, the environmental applications of stable metal isotopes for the tracing the sources of contamination and their importance in the development of these methods for the remediation of pollution and the protection of populations exposed to dangerous contamination.
Do not hesitate to help us improve our courses, all your feedback and constructive criticism are welcome, as well as your questions, in our forums or directly here, in comments!